Cologuard
Cologuard; Colon cancer screening - Cologuard; Stool DNA test - Cologuard; FIT-DNA stool test; Colon precancer screening - Cologuard
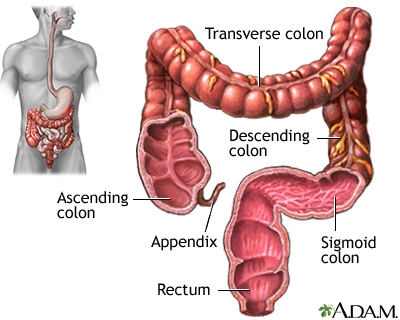
Cologuard is a screening test for colon and rectal cancer.
The colon sheds cells from its lining every day. These cells pass with the stool through the colon. Colon cancer cells may have DNA changes in certain genes. Cologuard detects the altered DNA. The presence of abnormal cells or blood in the stool may indicate cancer or precancer tumors.
Images
I Would Like to Learn About:
How the Test is Performed
The Cologuard testing kit for colon and rectal cancer must be ordered by your health care provider. It will be sent by mail to your address. You collect the sample at home and send it back to the lab for testing.
The Cologuard testing kit will contain a sample container, a tube, preserving liquid, labels, and instructions on how to collect the sample. When you are ready to have a bowel movement, use the Cologuard testing kit to collect your stool sample.
Read the instructions that come with the testing kit carefully. Wait until you are ready to have a bowel movement. Collect the sample only when it is possible to ship it within 24 hours. The sample must reach the lab in 72 hours (3 days).
DO NOT collect the sample if:
- You have diarrhea.
- You are menstruating.
- You have rectal bleeding due to hemorrhoids.
Follow these steps to collect the sample:
- Read all instructions that come with the kit.
- Use the brackets provided with the testing kit to fix the sample container on your toilet seat.
- Use the toilet as usual for your bowel movement.
- Try not to let urine get into the sample container.
- Do not put toilet paper into the sample container.
- Once your bowel movement is over, remove the sample container from the brackets and keep it on a flat surface.
- Follow instructions to collect a little sample in the tube provided with the testing kit.
- Pour the preserving liquid in the sample container and close the lid tightly.
- Label the tubes and the sample container according to the instructions, and place them in the box.
- Store the box at room temperature, away from direct sunlight and heat.
- Ship the box within 24 hours to the lab using the label provided.
The results of the test will be sent to your provider in two weeks.
How to Prepare for the Test
The Cologuard test does not require any preparation. You do not need to change your diet or medicines before the test.
How the Test will Feel
The test requires you to have a normal bowel movement. It will not feel any different from your regular bowel movements. You can collect the sample at your home privately.
Why the Test is Performed
The test is done to screen for colon and rectal cancer and abnormal growths (polyps) in the colon or rectum.
Your provider may suggest Cologuard testing once every 1 to 3 years starting at age 45 years. The test is recommended if you are age 45 to 75 years and have an average risk of colon cancer. This means that you do not have:
- Personal history of colon polyps and colon cancer
- Family history of colon cancer
- Inflammatory bowel disease (Crohn disease, ulcerative colitis)
Normal Results
The normal result (negative result) will indicate that:
- The test did not detect blood cells or altered DNA in your stool.
- You do not need further testing for colon cancer if you have an average risk of colon or rectal cancer.
What Abnormal Results Mean
Abnormal result (positive result) suggests that the test found some pre-cancer or cancer cells in your stool sample. However, the Cologuard test does not diagnose cancer. You will need further tests to make a diagnosis of cancer. Your provider will likely suggest a colonoscopy.
Risks
There is no risk involved in taking the sample for Cologuard test.
Screening tests carry a small risk of:
- False-positives (your test results are abnormal, but you do NOT have colon cancer or pre-malignant polyps)
- False-negatives (your test is normal, even when you have colon cancer)
Considerations
It is unclear yet whether the use of Cologuard will lead to better outcomes compared with other methods used to screen for colon and rectal cancer.
References
Bresalier RS. Colorectal cancer. In: Feldman M, Friedman LS, Brandt LJ, eds. Sleisenger and Fordtran's Gastrointestinal and Liver Disease. 11th ed. Philadelphia, PA: Elsevier; 2021:chap 127.
Eckmann JD, Ebner DW, Bering J, et al, eds. Multitarget stool DNA screening in clinical practice: high positive predictive value for colorectal neoplasia regardless of exposure to previous colonoscopy. Am J Gastroenterol. 2020;115(4):608-615. PMID: 32068535. pubmed.ncbi.nlm.nih.gov/32068535/.
NCCN clinical practice guidelines in oncology (NCCN Guidelines) colon cancer, version 2.2023. www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed August 29, 2023.
US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(19):1965-1977. PMID: 34003218 pubmed.ncbi.nlm.nih.gov/34003218/.
BACK TO TOPReview Date: 5/3/2023
Reviewed By: Michael M. Phillips, MD, Emeritus Professor of Medicine, The George Washington University School of Medicine, Washington, DC. Also reviewed by David C. Dugdale, MD, Medical Director, Brenda Conaway, Editorial Director, and the A.D.A.M. Editorial team.

Health Content Provider
06/01/2025
|
A.D.A.M., Inc. is accredited by URAC, for Health Content Provider (www.urac.org). URAC's accreditation program is an independent audit to verify that A.D.A.M. follows rigorous standards of quality and accountability. A.D.A.M. is among the first to achieve this important distinction for online health information and services. Learn more about A.D.A.M.'s editorial policy, editorial process and privacy policy. A.D.A.M. is also a founding member of Hi-Ethics. This site complied with the HONcode standard for trustworthy health information from 1995 to 2022, after which HON (Health On the Net, a not-for-profit organization that promoted transparent and reliable health information online) was discontinued. |
The information provided herein should not be used during any medical emergency or for the diagnosis or treatment of any medical condition. A licensed medical professional should be consulted for diagnosis and treatment of any and all medical conditions. Links to other sites are provided for information only -- they do not constitute endorsements of those other sites. © 1997- 2024 A.D.A.M., a business unit of Ebix, Inc. Any duplication or distribution of the information contained herein is strictly prohibited.