Drug-induced lupus erythematosus
Lupus - drug induced
Drug-induced lupus erythematosus is an autoimmune disorder that is triggered by a reaction to a medicine.
Images
I Would Like to Learn About:
Causes
Drug-induced lupus erythematosus is similar but not identical to systemic lupus erythematosus (SLE). It is an autoimmune disorder. This means your body attacks healthy tissue by mistake. It is caused by a reaction to a medicine. Related conditions are drug-induced cutaneous lupus and drug-induced ANCA vasculitis.
The most common medicines known to cause drug-induced lupus erythematosus are:
- Isoniazid
- Hydralazine
- Procainamide
- Tumor-necrosis factor (TNF) alpha inhibitors (such as etanercept, infliximab and adalimumab)
- Minocycline
- Quinidine
Other less common drugs may also cause the condition. These may include:
- Anti-seizure medicines
- Capoten
- Chlorpromazine
- Methyldopa
- Sulfasalazine
- Levamisole, typically as a contaminant of cocaine
Cancer immunotherapy drugs such as pembrolizumab can also cause a variety of autoimmune reactions including drug-induced lupus.
Symptoms of drug-induced lupus tend to occur after taking the drug for at least 3 to 6 months.
Symptoms
Symptoms may include:
- Fever
- General ill feeling (malaise)
- Joint pain
- Joint swelling
- Loss of appetite
- Pleuritic chest pain (sharp pain that is worse with breathing in)
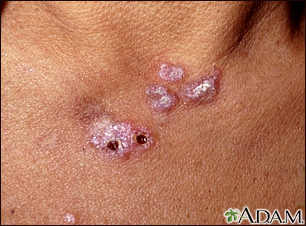
- Skin rash on areas exposed to sunlight
Exams and Tests
The health care provider will do a physical exam and listen to your chest with a stethoscope. The provider may hear a sound called a heart friction rub or pleural friction rub.
A skin exam shows a rash.
Joints may be swollen and tender.
Tests that may be done include:
- Antihistone antibody
- Antinuclear antibody (ANA) panel
- Antineutrophil cytoplasmic antibody (ANCA) panel
- Complete blood count (CBC) with differential
- Comprehensive chemistry panel
- Urinalysis
A chest x-ray may show signs of pleuritis or pericarditis (inflammation around the lining of the lung or heart). An ECG may show that the heart is affected.
Treatment
Most of the time, symptoms go away within weeks after stopping the medicine that caused the condition.
Treatment may include:
- Nonsteroidal anti-inflammatory drugs (NSAIDs) to treat arthritis and pleurisy
- Corticosteroid creams to treat skin rashes
- Antimalarial drugs (hydroxychloroquine) to treat skin and arthritis symptoms
If the condition is affecting your heart, kidney, or nervous system, you may be prescribed high doses of corticosteroids (prednisone, methylprednisolone) and immune system suppressants (azathioprine or cyclophosphamide). This is rare.
When the disease is active, you should wear protective clothing and sunglasses to guard against too much sun.
Outlook (Prognosis)
Most of the time, drug-induced lupus erythematosus is not as severe as SLE. The symptoms often go away within a few days to weeks after stopping the medicine you were taking. Rarely, kidney inflammation (nephritis) can develop with drug-induced lupus caused by TNF inhibitors or with ANCA vasculitis due to hydralazine or levamisole. Nephritis may require treatment with prednisone and immunosuppressive medicines.
Avoid taking the drug that caused the reaction in future. Symptoms are likely to return if you do so.
Possible Complications
Complications may include:
- Infection
- Thrombocytopenic purpura -- bleeding near the skin surface, resulting from a low number of platelets in the blood
- Hemolytic anemia
- Myocarditis
- Pericarditis
- Nephritis
When to Contact a Medical Professional
Contact your provider if:
- You develop new symptoms when taking any of the medicines listed above.
- Your symptoms do not get better after you stop taking the medicine that caused the condition.
Prevention
Watch for signs of a reaction if you are taking any of the drugs that can cause this problem.
Related Information
Autoimmune disordersSystemic lupus erythematosus
Chronic
Immune response
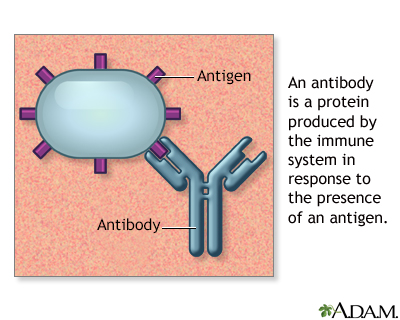
Antibody
Arthritis
Systemic
Lupus nephritis
Hemolytic anemia
Myocarditis
References
Benfaremo D, Manfredi L, Luchetti MM, Gabrielli A. Musculoskeletal and rheumatic diseases induced by immune checkpoint inhibitors: a review of the literature. Curr Drug Saf. 2018;13(3):150-164. PMID: 29745339 pubmed.ncbi.nlm.nih.gov/29745339/.
Radhakrishnan J, Perazella MA. Drug-induced glomerular disease: attention required. Clin J Am Soc Nephrol. 2015;10(7):1287-1290. PMID: 25876771 pubmed.ncbi.nlm.nih.gov/25876771/.
Richardson B. Drug-induced lupus. In: Hochberg MC, Gravallese EM, Smolen JS, van der Heijde D, Weinblatt ME, Weisman MH, eds. Rheumatology. 8th ed. Philadelphia, PA: Elsevier; 2023:chap 140.
Rubin RL. Drug-induced lupus. Expert Opin Drug Saf. 2015;14(3):361-378. PMID: 25554102 pubmed.ncbi.nlm.nih.gov/25554102/.
Vaglio A, Grayson PC, Fenaroli P, et al. Drug-induced lupus: traditional and new concepts. Autoimmun Rev. 2018;17(9):912-918. PMID: 30005854 pubmed.ncbi.nlm.nih.gov/30005854/.
BACK TO TOPReview Date: 4/30/2023
Reviewed By: Neil J. Gonter, MD, Assistant Professor of Medicine, Columbia University, New York, NY, and private practice specializing in Rheumatology at Rheumatology Associates of North Jersey, Teaneck, NJ. Review provided by VeriMed Healthcare Network. Also reviewed by David C. Dugdale, MD, Medical Director, Brenda Conaway, Editorial Director, and the A.D.A.M. Editorial team.

Health Content Provider
06/01/2025
|
A.D.A.M., Inc. is accredited by URAC, for Health Content Provider (www.urac.org). URAC's accreditation program is an independent audit to verify that A.D.A.M. follows rigorous standards of quality and accountability. A.D.A.M. is among the first to achieve this important distinction for online health information and services. Learn more about A.D.A.M.'s editorial policy, editorial process and privacy policy. A.D.A.M. is also a founding member of Hi-Ethics. This site complied with the HONcode standard for trustworthy health information from 1995 to 2022, after which HON (Health On the Net, a not-for-profit organization that promoted transparent and reliable health information online) was discontinued. |
The information provided herein should not be used during any medical emergency or for the diagnosis or treatment of any medical condition. A licensed medical professional should be consulted for diagnosis and treatment of any and all medical conditions. Links to other sites are provided for information only -- they do not constitute endorsements of those other sites. © 1997- 2025 A.D.A.M., a business unit of Ebix, Inc. Any duplication or distribution of the information contained herein is strictly prohibited.