Pneumococcal conjugate vaccine - what you need to know
Content below is taken in its entirety from the CDC Information Statement (VIS): www.cdc.gov/vaccines/hcp/current-vis/pneumococcal-conjugate.html
Information
1. Why get vaccinated?
Pneumococcal conjugate vaccine can prevent pneumococcal disease.
Pneumococcal disease refers to any illness caused by pneumococcal bacteria. These bacteria can cause many types of illnesses, including:
- Pneumonia, (infection of the lungs)
- Ear infections
- Sinus infections
- Meningitis (infection of the tissue covering the brain and spinal cord)
- Bacteremia (bloodstream infection)
Anyone can get pneumococcal disease, but young children, older adults, and people with certain risk factors are at the highest risk.
Most pneumococcal infections are mild. However, some can result in long-term problems, such as brain damage or hearing loss. Meningitis, bacteremia, and pneumonia caused by pneumococcal disease can lead to death.
2. Pneumococcal conjugate vaccine
Pneumococcal conjugate vaccine helps protect against bacteria that cause pneumococcal disease. There are several pneumococcal conjugate vaccines (PCVs). The specific PCV and number of doses recommended are based on a person's age, vaccination history, and medical status. Your health care provider can help you determine which type of PCV, and how many doses, should be received.
- Infants and young children usually need 4 doses of PCV. These doses are recommended, at 2, 4, 6, and 12-15 months of age.
- Certain older children and adolescents who did not receive the recommended doses as infants or young children need PCV. This depends on age and medical conditions, or other risk factors.
- Adults 19 through 49 years old who have not received PCV and have certain medical conditions or other risk factors should receive PCV. Some adults in this group who have already received PCV might be recommended to receive another dose.
- Adults 50 years or older who have not previously received PCV should receive a PCV vaccine. Some adults in this group who have already received PCV might be recommended to receive another dose.
3. Talk with your health care provider
Tell your vaccination provider if the person getting the vaccine:
- Has had an allergic reaction after a previous dose of any type of PCV, or to any vaccine containing diphtheria toxoid (for example, DTaP), or has any severe, life-threatening allergies.
In some cases, your health care provider may decide to postpone PCV until a future visit.
People with minor illnesses, such as a cold, may be vaccinated. People who are moderately or severely ill should usually wait until they recover.
Your health care provider can give you more information.
4. Risks of a vaccine reaction
- Redness, swelling, pain, or tenderness where the shot is given; fever; loss of appetite; fussiness (irritability); tiredness; headache; muscle aches; joint pain; or chills can happen after pneumococcal conjugate vaccination.
Young children may be at increased risk for seizures caused by fever after a pneumococcal conjugate vaccine if it is administered at the same time as inactivated influenza vaccine. Ask your health care provider for more information.
People sometimes faint after medical procedures, including vaccination. Tell your provider if you feel dizzy or have vision changes or ringing in the ears.
As with any medicine, there is a very remote chance of a vaccine causing a severe allergic reaction, other serious injury, or death.
5. What if there is a serious problem?
An allergic reaction could occur after the vaccinated person leaves the clinic. If you see signs of a severe allergic reaction (hives, swelling of the face and throat, difficulty breathing, a fast heartbeat, dizziness, or weakness), call 9-1-1 and get the person to the nearest hospital.
For other signs that concern you, call your health care provider.
Adverse reactions should be reported to the Vaccine Adverse Event Reporting System (VAERS). Your health care provider will usually file this report, or you can do it yourself. Visit the VAERS website or call 1-800-822-7967. VAERS is only for reporting reactions, and VAERS staff members do not give medical advice.
6. The National Vaccine Injury Compensation Program
The National Vaccine Injury Compensation Program (VICP) is a federal program that was created to compensate people who may have been injured by certain vaccines. Claims regarding alleged injury or death due to vaccination have a time limit for filing, which may be as short as two years. Visit the VICP website or call 1-800-338-2382 to learn about the program and about filing a claim.
7. How can I learn more?
- Ask your health care provider.
- Call your local or state health department.
- Visit the website of the Food and Drug Administration (FDA) for package inserts and additional information.
Contact the Centers for Disease Control and Prevention (CDC):
- Call 1-800-232-4636 (1-800-CDC-INFO) or
- Visit CDC's vaccine website.
References
Centers for Disease Control and Prevention website. Pneumococcal Conjugate VIS (Interim). www.cdc.gov/vaccines/hcp/current-vis/pneumococcal-conjugate.html. Updated May 29, 2025. Accessed June 4, 2025.
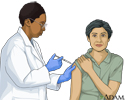
Pneumococcal vaccine is an immunization against Streptococcus pneumoniae, a bacterium that frequently causes meningitis and pneumonia in the elderly, and people with chronic illnesses. Pneumococcal pneumonia accounts for 10% to 25% of all pneumonias. The pneumococcal vaccine is recommended for children ages 5 and younger and adults ages 50 and older. Ideally, children should receive 4 doses starting at age 2 months. Most adults will receive 1 or more shots, depending on age and vaccine history. Adults and children at higher risk may need additional shots. Talk with your health care provider about the right vaccine schedule for you.
Pneumococcal vaccine
illustration
Vaccines are used to boost your immune system and prevent many diseases, some of which are serious or life-threatening. Vaccines “teach“ your body how to defend itself when germs, such as viruses or bacteria, invade it. After exposure to the vaccine, your immune system learns to recognize and attack the viruses or bacteria if you are exposed to them later in life. As a result, you will not become ill. Or, if you do get the illness, you will likely have a milder infection. Vaccines are very safe and very effective at protecting against certain serious diseases.
Vaccines
illustration
Pneumococcal vaccine is an immunization against Streptococcus pneumoniae, a bacterium that frequently causes meningitis and pneumonia in the elderly, and people with chronic illnesses. Pneumococcal pneumonia accounts for 10% to 25% of all pneumonias. The pneumococcal vaccine is recommended for children ages 5 and younger and adults ages 50 and older. Ideally, children should receive 4 doses starting at age 2 months. Most adults will receive 1 or more shots, depending on age and vaccine history. Adults and children at higher risk may need additional shots. Talk with your health care provider about the right vaccine schedule for you.
Pneumococcal vaccine
illustration
Vaccines are used to boost your immune system and prevent many diseases, some of which are serious or life-threatening. Vaccines “teach“ your body how to defend itself when germs, such as viruses or bacteria, invade it. After exposure to the vaccine, your immune system learns to recognize and attack the viruses or bacteria if you are exposed to them later in life. As a result, you will not become ill. Or, if you do get the illness, you will likely have a milder infection. Vaccines are very safe and very effective at protecting against certain serious diseases.
Vaccines
illustration
- Immunizations - InDepth(In-Depth)
- Otitis media(Alt. Medicine)
- Asthma in children and adolescents - InDepth(In-Depth)
Review Date: 4/9/2024
Reviewed By: Frank D. Brodkey, MD, FCCM, Associate Professor, Section of Pulmonary and Critical Care Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI. Also reviewed by David C. Dugdale, MD, Medical Director, Brenda Conaway, Editorial Director, and the A.D.A.M. Editorial team. Editorial update 06/04/2025.