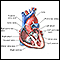
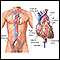
Hypoplastic left heart syndrome
HLHS; Congenital heart - hypoplastic left heart; Cyanotic heart disease - hypoplastic left heartHypoplastic left heart syndrome occurs when parts of the left side of the heart (mitral valve, left ventricle, aortic valve, and aorta) do not develop completely. The condition is present at birth (congenital).
Causes
Hypoplastic left heart is a rare type of congenital heart disease. It is more common in males than in females.
As with most congenital heart defects, there is no known cause. About 10% of babies with hypoplastic left heart syndrome also have other birth defects. It is also associated with some genetic diseases such as Turner syndrome, Jacobsen syndrome, trisomy 13 and 18.
The problem develops before birth when the left ventricle and other structures do not grow properly, including the:
- Aorta (the blood vessel that carries oxygen-rich blood from the left ventricle to the entire body)
- Entrance and exit of the ventricle
- Mitral and aortic valves
This causes the left ventricle and aorta to be poorly developed, or hypoplastic. In most cases, the left ventricle and aorta are much smaller than normal.
In babies with this condition, the left side of the heart is unable to send enough blood to the body. As a result, the right side of the heart must maintain the circulation for both the lungs and the body. The right ventricle can support the circulation to both the lungs and the body for a while, but this extra workload eventually causes the right side of the heart to fail.
The only possibility of survival is a connection between the right and the left side of the heart, or between the arteries and pulmonary arteries (the blood vessels that carry blood to the lungs). Babies are normally born with two of these connections:
-
Foramen ovale (a hole between the right and left atrium)
Foramen
Patent foramen ovale (PFO) is a hole between the left and right atria (upper chambers) of the heart. This hole exists in everyone before birth, but ...
 ImageRead Article Now Book Mark Article
ImageRead Article Now Book Mark Article -
Ductus arteriosus (a small blood vessel that connects the aorta to the pulmonary artery)
Ductus arteriosus
Patent ductus arteriosus (PDA) is a condition in which the ductus arteriosus does not close. The word "patent" means open. The ductus arteriosus is ...
 ImageRead Article Now Book Mark Article
ImageRead Article Now Book Mark Article
Both connections normally close on their own a few days after birth.
In babies with hypoplastic left heart syndrome, blood leaving the right side of the heart through the pulmonary artery travels through the ductus arteriosus to the aorta. This is the only way for blood to get to the body. If the ductus arteriosus is allowed to close in a baby with hypoplastic left heart syndrome, the baby may quickly die because no blood will be pumped to the body. Babies with known hypoplastic left heart syndrome are usually started on a medicine to keep the ductus arteriosus open.
Because there is little or no flow out of the left heart, blood returning to the heart from the lungs needs to pass through the foramen ovale or an atrial septal defect (a hole connecting the collecting chambers on the left and right sides of the heart) back to the right side of the heart. If there is no foramen ovale, or if it is too small, the baby could die. Babies with this problem have the hole between their atria opened, either with surgery or using a thin, flexible tube (heart catheterization).
Symptoms
At first, a newborn with hypoplastic left heart may appear normal. Symptoms may occur in the first few hours of life, although it may take up to a few days to develop symptoms. These symptoms may include:
- Bluish color to the skin (cyanosis) due to low oxygen level in the blood
Cyanosis
A bluish color to the skin or mucous membrane is usually due to a lack of oxygen in the blood. The medical term is cyanosis.
 ImageRead Article Now Book Mark Article
ImageRead Article Now Book Mark Article - Cold hands and feet (extremities)
- Lethargy
- Poor pulse
- Poor suckling and feeding
- Pounding heart
- Rapid breathing
- Shortness of breath
In healthy newborns, bluish color in the hands and feet is a response to cold (this reaction is called peripheral cyanosis).
A bluish color in the chest or abdomen, lips, and tongue is abnormal (called central cyanosis). It is a sign that there is not enough oxygen in the blood. Central cyanosis often increases with crying.
Exams and Tests
A physical exam may show signs of heart failure:
- Faster than normal heart rate
- Lethargy
- Liver enlargement
- Rapid breathing
Also, the pulse at various locations (wrist, groin, and others) may be very weak. There are often (but not always) abnormal heart sounds when listening to the chest.
Tests may include:
-
Cardiac catheterization
Cardiac catheterization
Cardiac catheterization involves passing a thin flexible tube (catheter) into the right or left side of the heart. The catheter is most often insert...
 ImageRead Article Now Book Mark Article
ImageRead Article Now Book Mark Article -
ECG (electrocardiogram)
ECG
An electrocardiogram (ECG) is a test that records the electrical activity of the heart.
 ImageRead Article Now Book Mark Article
ImageRead Article Now Book Mark Article -
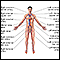
Echocardiogram
Echocardiogram
An echocardiogram is a test that uses sound waves to create pictures of the heart. The picture and information it produces is more detailed than a s...
 ImageRead Article Now Book Mark Article
ImageRead Article Now Book Mark Article -
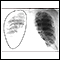
X-ray of the chest
X-ray of the chest
A chest x-ray is an x-ray of the chest, lungs, heart, large arteries, ribs, and diaphragm.
 ImageRead Article Now Book Mark Article
ImageRead Article Now Book Mark Article
Treatment
Once the diagnosis of hypoplastic left heart is made, the baby will be admitted to the neonatal intensive care unit. A breathing machine (ventilator) may be needed to help the baby breathe. A medicine called prostaglandin E1 is used to keep blood circulating to the body by keeping the ductus arteriosus open.
These measures do not solve the problem. The condition always requires surgery.
The first surgery, called the Norwood operation, occurs within the baby's first few days of life. The Norwood procedure consists of building a new aorta by:
- Using the pulmonary valve and artery
- Connecting the hypoplastic old aorta and coronary arteries to the new aorta
- Removing the wall between the atria (atrial septum)
- Making an artificial connection from either the right ventricle or a bodywide artery to the pulmonary artery to maintain blood flow to the lungs (called a shunt)
A variation of the Norwood procedure, called the Sano procedure, may be used. This procedure creates a right ventricle to pulmonary artery connection.
Afterward, the baby goes home in most cases. The child will need to take daily medicines and be closely followed by a pediatric cardiologist, who will determine when the second stage of surgery should be done.
Stage II of the operation is called the Glenn shunt or hemi-Fontan procedure. It is also referred to as a cavopulmonary shunt. This procedure connects the major vein carrying the blood that is depleted of oxygen from the top half of the body (the superior vena cava) directly to blood vessels to the lungs (pulmonary arteries) to get oxygen. The surgery is most often done when the child is 4 to 6 months of age.
During stages I and II, the child may still appear somewhat blue (cyanotic).
Stage III, the final step, is called the Fontan procedure. The rest of the veins that carry blue blood from the body (the inferior vena cava) are connected directly to the blood vessels to the lungs. The right ventricle now serves only as the pumping chamber for the body (no longer the lungs and the body). This surgery is usually performed when the baby is 18 months to 4 years old. After this final step, the child is no longer cyanotic and has a normal oxygen level in the blood.
Some people may need more surgeries in their 20s or 30s if they develop hard to control arrhythmias or other complications of the Fontan procedure.
Some doctors consider heart transplantation an alternative to the 3 step surgery. But there are few donated hearts available for small infants.
Outlook (Prognosis)
If left untreated, hypoplastic left heart syndrome is fatal. Survival rates for the staged repair continue to rise as surgery techniques and care after surgery improve. Survival after the first stage is more than 75%. Children who survive their first year have a very good chance for long-term survival.
The child's outcome after surgery depends on the size and function of the right ventricle.
Possible Complications
Complications include:
- Blockage of the artificial shunt
- Blood clots that can lead to stroke or pulmonary embolism
- Long-term (chronic) diarrhea (from a disease called protein-losing enteropathy)
- Fluid in the abdomen (ascites) and in the lungs (pleural effusion)
Ascites
Ascites is the build-up of fluid in the space between the lining of the abdomen and abdominal organs.
 ImageRead Article Now Book Mark Article
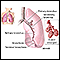
ImageRead Article Now Book Mark ArticlePleural effusion
A pleural effusion is a buildup of fluid between the layers of tissue that line the lungs and chest cavity.
 ImageRead Article Now Book Mark Article
ImageRead Article Now Book Mark Article -
Heart failure
Heart failure
Heart failure is a condition in which the heart is no longer able to pump oxygen-rich blood to the rest of the body efficiently. This causes symptom...
 ImageRead Article Now Book Mark Article
ImageRead Article Now Book Mark Article - Irregular, fast heart rhythms (arrhythmias)
- Strokes and other nervous system complications
- Neurological impairment
- Sudden death
When to Contact a Medical Professional
Contact your health care provider right away if your infant:
- Eats less (decreased feeding)
- Has blue (cyanotic) skin
- Has new changes in breathing patterns
Prevention
There is no known prevention for hypoplastic left heart syndrome. As with many congenital diseases, the causes of hypoplastic left heart syndrome are uncertain and have not been linked to a mother's disease or behavior.
Women who plan to become pregnant should be immunized against rubella if they are not already immune. Rubella infection in a pregnant woman can cause congenital heart disease.
Women who are pregnant should get good prenatal care:
- Avoid alcohol and illegal drugs during pregnancy.
- Tell your health care provider that you are pregnant before taking any new medicines.
- Have a blood test early in your pregnancy to see if you are immune to rubella. If you are not immune, avoid any possible exposure to rubella and get vaccinated right after delivery.
- Pregnant women who have diabetes should try to get good control over their blood sugar level.
References
Bernstein D. Cyanotic congenital heart disease: evaluation of the critically ill neonate with cyanosis and respiratory distress. In: Kliegman RM, St. Geme JW, Blum NJ, Shah SS, Tasker RC, MBBS, Wilson KM, eds. Nelson Textbook of Pediatrics. 21st ed. Philadelphia, PA: Elsevier; 2020:chap 456.
Valente AM, Dorfman AL, Babu-Narayan SV, Kreiger EV. Congenital heart disease in the adolescent and adult. In: Libby P, Bonow RO, Mann DL, Tomaselli GF, Bhatt DL, Solomon SD, eds. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 12th ed. Philadelphia, PA: Elsevier; 2022:chap 82.
Well A, Fraser CD. Congenital heart disease. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery. 21st ed. St Louis, MO: Elsevier; 2022:chap 59.
-
Heart - section through the middle - illustration
The interior of the heart is composed of valves, chambers, and associated vessels.
Heart - section through the middle
illustration
-
Heart - front view - illustration
The external structures of the heart include the ventricles, atria, arteries and veins. Arteries carry blood away from the heart while veins carry blood into the heart. The vessels colored blue indicate the transport of blood with relatively low content of oxygen and high content of carbon dioxide. The vessels colored red indicate the transport of blood with relatively high content of oxygen and low content of carbon dioxide.
Heart - front view
illustration
-
Hypoplastic left heart syndrome - illustration
Hypoplastic left heart syndrome is a congenital heart condition that occurs during the development of the heart in the mother's womb. During the heart's development, parts of the left side of the heart (mitral valve, left ventricle aortic valve, and aorta) do not develop completely. In patients with this condition, the left side of the heart is unable to send enough blood to the body.
Hypoplastic left heart syndrome
illustration
-
Heart - section through the middle - illustration
The interior of the heart is composed of valves, chambers, and associated vessels.
Heart - section through the middle
illustration
-
Heart - front view - illustration
The external structures of the heart include the ventricles, atria, arteries and veins. Arteries carry blood away from the heart while veins carry blood into the heart. The vessels colored blue indicate the transport of blood with relatively low content of oxygen and high content of carbon dioxide. The vessels colored red indicate the transport of blood with relatively high content of oxygen and low content of carbon dioxide.
Heart - front view
illustration
-
Hypoplastic left heart syndrome - illustration
Hypoplastic left heart syndrome is a congenital heart condition that occurs during the development of the heart in the mother's womb. During the heart's development, parts of the left side of the heart (mitral valve, left ventricle aortic valve, and aorta) do not develop completely. In patients with this condition, the left side of the heart is unable to send enough blood to the body.
Hypoplastic left heart syndrome
illustration
Review Date: 10/23/2023
Reviewed By: Michael A. Chen, MD, PhD, Associate Professor of Medicine, Division of Cardiology, Harborview Medical Center, University of Washington Medical School, Seattle, WA. Also reviewed by David C. Dugdale, MD, Medical Director, Brenda Conaway, Editorial Director, and the A.D.A.M. Editorial team.