Breast cancer - InDepth
Cancer - breast - InDepth; Carcinoma - ductal - InDepth; Carcinoma - lobular - InDepth; DCIS - InDepth; LCIS - InDepth; HER2-positive breast cancer - InDepth; ER-positive breast cancer - InDepth; Ductal carcinoma in situ - InDepth; Lobular carcinoma in situ - InDepthAn in-depth report on the causes, diagnosis, treatment, and prevention of breast cancer.
Highlights
Breast Cancer Screening
Mammography is the best screening method for detecting breast cancer when it is in its earliest and most treatable stages. However, debate continues on whether mammograms contribute to overdiagnosis and overtreatment of breast cancer.
Much of the debate focuses on when women should begin to have mammograms and how frequently they should receive them. Experts continue to review new information on these issues.
The current recommendations on breast cancer screening vary greatly among the different professional medical organizations. Therefore, the decision to start screening mammography should be made individually, after discussing with your health care provider the risks and benefits of mammography, your personal risk factors for breast cancer, and your feelings and preferences about screening.
Genetic Risk Screening
Mutations in the genes called BRCA1 and BRCA2 are among the strongest risk factors for breast and ovarian cancers. The United States Preventive Service Task Force (USPSTF) recommends testing for BRCA genetic mutations in women whose family history screening suggests a high risk for BRCA mutations. Women who do not have family history associated with increased risk for BRCA mutations do not need to be tested.
Your provider may screen your risk for these genes using a questionnaire. Having several first-degree or second-degree relatives who have had breast, ovarian, fallopian tube, or peritoneal cancers is an indication of risk.
If you are at risk, you may be referred to a genetic counselor who can discuss whether you should be tested for these genetic mutations.
Introduction
Breast cancer is cancer that begins in the cells of the breast. When cells grow at an abnormally fast rate, they create a mass of tissue called a tumor.
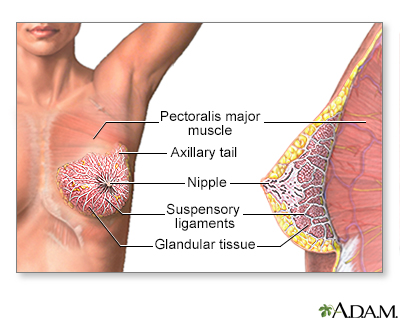
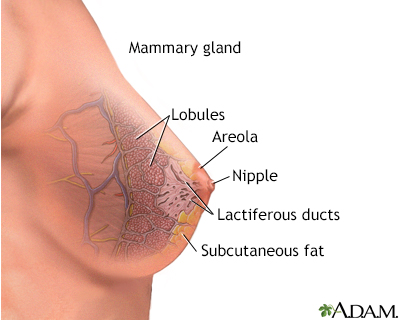
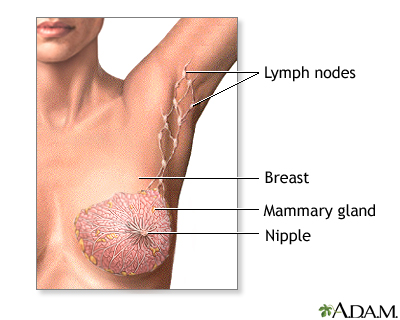
The breast is divided into sections called lobes. Each lobe is divided into a collection of milk-producing glands called lobules. Lobules secrete milk into tunnels of ducts, which are tiny tubes that carry milk through the breast to the nipple. Breast cancer usually originates in the lobules or the ducts.
Female breasts are also known as mammary glands because they are able to produce milk.
Breast cancer can also occur in men, although with a much lower incidence than in women.
Breast cancer is classified as either:
- Non-invasive, also called in situ. The cancer is confined to the duct or lobule.
- Invasive, also called infiltrating. The cancer has spread out of the duct or lobule into neighboring breast tissue or beyond.
Non-Invasive Breast Cancer
Non-invasive breast cancers include:
- Ductal carcinoma in situ (DCIS). Cancer cells in the lining of the milk duct. DCIS is considered "pre-invasive" or "pre-cancer." Although DCIS is non-invasive, if left untreated it may progress to invasive ductal breast cancer. DCIS is the most common type of non-invasive breast cancer.
- Lobular carcinoma in situ (LCIS). The presence of abnormal cells in the lobules of the breast. Although LCIS is technically not a cancer, it increases the risk for invasive breast cancer.
Invasive Breast Cancer
Invasive (infiltrating) breast cancer occurs when cancer cells penetrate beyond the wall of a duct or lobule into surrounding connective tissue in the breast. If left untreated, invasive breast cancers can spread to other parts of the body through the bloodstream or lymphatic system.
Invasive breast cancers include:
- Invasive ductal carcinoma. Starts and spreads from the milk duct. It is the most common type of invasive breast cancer. About 8 in 10 women with invasive breast cancer have infiltrating ductal carcinoma.
- Invasive lobular carcinoma. Starts and spreads from the milk-producing lobule It is the second most common type of invasive breast cancer. About 1 in 10 women with invasive breast cancer have infiltrating lobular carcinoma.
- There are other less common breast cancers that are not discussed in this report.
Risk Factors
Breast cancer is the most common cancer in women worldwide. About 1.7 million women globally are diagnosed with invasive breast cancer each year. In the United States, about 250,000 women are diagnosed with invasive breast cancer each year, and 1 in 8 women (about 12%) will develop invasive breast cancer in their lifetime. Although breast cancer in men is rare, about 2,000 men in the United States are diagnosed each year with invasive breast cancer.
There are many different risk factors for breast cancer.
Age
The risk for breast cancer increases with age. According to the American Cancer Society (ACS), about 1 in 8 cases of invasive breast cancer are found in women younger than age 45, while 2 in 3 cases of invasive breast cancer are found in women ages 55 and older.
Race and Ethnicity
In the United States, breast cancer is more common among white women compared with African American, Asian, Hispanic, or Native American women. However, African American women tend to have more aggressive types of breast tumors and are more likely to die from breast cancer than women of other races.
Breast cancer is also more prevalent among Jewish women of Eastern European (Ashkenazi) descent.
Family and Personal History
Women who have a family history of breast cancer are at increased risk for developing breast cancer themselves. Having a first-degree relative (mother, sister, or daughter) who has been diagnosed with breast cancer nearly doubles the risk of developing breast cancer.
Women who have had ovarian cancer are at increased risk for developing breast cancer. A personal history of breast cancer increases the risk of developing a new cancer in the same or other breast.
Genetic Factors
About 5% to 10% of breast cancer cases are due to inherited genetic mutations.
BRCA Genes
Inherited mutations in genes known as BRCA1 or BRCA2 are responsible for most cases of hereditary breast cancers and ovarian cancers. Women with BRCA1 mutations have a 55% to 65% lifetime risk for breast cancer. For BRCA2 mutations, the lifetime risk for breast cancer is around 45%.
BRCA gene mutations are present in only about 0.5% of the overall population. Jewish women of Eastern European (Ashkenazi) descent have a higher prevalence (2.5%) of BRCA gene mutations. However, BRCA gene mutations can occur in women of all ethnic backgrounds.
Screening for BRCA Genes
The USPSTF recommends BRCA screening for women at high risk due to family history. Women who do not have a family history of breast cancer have a low probability of inheriting BRCA genetic mutations and do not need to be tested.
Risk assessment is based on family history on both the maternal and paternal sides. The following personal or family history factors may indicate increased risk for BRCA mutations:
- Having several first-degree (mother, sister, daughter) or second-degree (aunt, grandmother) relatives who have had breast, ovarian, fallopian, or peritoneal cancer
- Breast cancer diagnosed before age 50
- Triple negative breast cancer
- Cancer in both breasts
- Breast cancer in a male relative
- Ashkenazi Jewish or Eastern European ancestry
Your health care provider can screen you using a questionnaire that evaluates your family and personal medical history, and other factors. If your provider decides you are at risk, you may be referred to a genetic counselor who can discuss whether you should be tested for the BRCA1 and BRCA2 mutations.
Other Genetic Mutations
Other genes more commonly associated with increased hereditary breast cancer risk include:
- p53
- CHEK2
- ATM
- PTEN
- CDH1
- STK11
- PALB2
Researchers are continuing to make progress in discovering molecular subtypes of breast cancer.
Exposure to Estrogen
Because growth of breast tissue is dependent on estrogen, the more estrogen a woman is exposed to over her lifetime, the higher her risk for breast cancer.
Duration of Estrogen Exposure
Early menarche (first menstrual period) or later menopause may slightly increase a woman's risk for breast cancer.
Pregnancy
Women who have never had children or who had their first child after age 30 may have a slightly increased breast cancer risk. Having children at an early age, and having multiple pregnancies, reduces breast cancer risk. Scientific evidence shows there is no association between abortion and increased breast cancer risk.
Breastfeeding
Study results have been mixed on whether breastfeeding decreases breast cancer risk. Breastfeeding reduces a woman's total number of menstrual cycles and thereby estrogen exposure, which may account for its possible protective effects. Some studies suggest that the longer a woman breast-feeds, the lower her risk for breast cancer, and that breastfeeding may be especially protective for women with a family history of breast cancer.
Birth Control Pills
Because most oral contraceptives contain estrogen, there have been questions on whether they can increase cancer risk. Evidence indicates that current or former oral contraceptive use does not significantly increase breast cancer risk. Women who have used oral contraceptives may have slightly more risk for breast cancer than women who have never used them, but this risk declines once a woman stops using birth control pills.
Hormone Therapy
Hormone therapy (HT) for menopausal symptoms uses either estrogen alone or estrogen and progestogen:
- Estrogen-progestogen therapy (EPT) is used by women who have a uterus, because estrogen alone can increase the risk for uterine cancer. EPT significantly increases the risk for developing and dying from breast cancer, especially when used for more than 5 years. EPT may also increase the risk for heart attacks, stroke, and deep vein thrombosis.
- Estrogen-only therapy (ET) is prescribed for women who have had a hysterectomy and do not have a uterus. ET does not appear to increase the risk for breast cancer as much as EPT. Additionally, prolonged used of ET can increase the risk for other health problems including blood clots, heart attack, stroke, and possibly ovarian cancer.
- Hormone therapy (EPT or ET) should not be used by women at high risk for breast cancer.
In general, most doctors recommend that women use HT only for short-term (1 to 2 years) relief of menopausal symptoms. Current guidelines advise initiating hormone therapy around the time of menopause when women are in their 40s or 50s. Starting HT past age 59 may increase the risk for breast cancer and other health problems.
Women who take HT should be aware that they need regular mammogram screenings, because HT increases breast cancer density, making mammograms more difficult to read.
Breast Conditions
Certain breast conditions may increase the risk for breast cancer, such as:
- Dense breast tissue. Dense breasts make mammograms more difficult to read, which increases the likelihood of missing early signs of cancer.
- Benign proliferative breast disease, or unusual cell growth known as atypical hyperplasia.
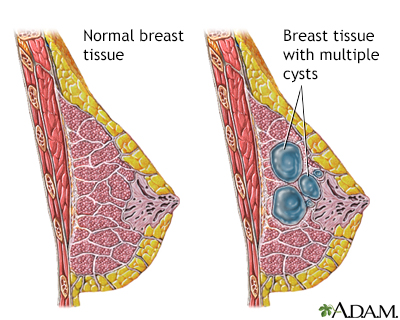
Common benign breast abnormalities pose little or no risk for breast cancer. These conditions include:
- Cysts
- Fibroadenoma
- Temporary breast pain
Still, check with your health care provider if you notice any changes in your breasts.
Physical Characteristics
Obesity increases the overall risk for cancer. Obesity is a risk factor for estrogen receptor-positive types of breast cancer. High amounts of fat tissue increase estrogen levels in the body.
Estrogen is also involved in building bone mass. Therefore, women with heavy, dense bones are likely to have higher estrogen levels and to be at greater risk for breast cancer.
Environmental Factors
Exposure to Estrogen-like Industrial Chemicals
Chemicals with estrogen-like effects, called xenoestrogens, have been under suspicion of increasing the risk for breast cancer for years. At this time, evidence of any causal association is very weak.
Exposure to Diethylstilbestrol
Women who took diethylstilbestrol (DES) to prevent miscarriage have a slightly increased risk for breast cancer. There may also be a slightly increased risk for their daughters (commonly called "DES daughters"), who were exposed to the drug in utero.
Radiation Exposure
Heavy exposure to radiation is a significant risk factor for breast cancer. Girls who receive high-dose radiation therapy for cancer face an increased risk for breast cancer in adulthood. Low-dose radiation exposure before age 20 may increase the risk for women with BRCA mutations. Women should avoid unnecessary and excessive exposure to medical radiation, including x-rays and CT scans.
Lifestyle Factors
Alcohol consumption is a risk factor for breast cancer. Women who have 2 or more drinks a day have a 50% increase in their breast cancer risk compared to women who do not drink.
Disproven Risk Factors
Antiperspirants or use of deodorants after shaving have not been linked with any higher risk for breast cancer. There is also no evidence that wearing bras increases breast cancer risk. Stress has been ruled out as a risk factor for breast cancer development or recurrence.
Prevention and Lifestyle Factors
Exercise
Regular exercise may protect against breast cancer. Exercise can help reduce body fat, which in turn lowers levels of cancer-promoting hormones, such as estrogen. The ACS recommends engaging in at least 150 minutes of moderate activity, 75 minutes of intense activity, or a combination thereof each week, preferably spread out throughout the week.
Exercise can also help women who have been diagnosed with breast cancer and may help reduce the risk of breast cancer recurrence. Studies indicate that both aerobic and weight training exercises benefit the body and the mind and improve quality of life for breast cancer survivors.
Dietary Factors
Despite much research on the association between diet and breast cancer, there is still little consensus. The best advice is to eat a well-balanced diet and avoid focusing on one "cancer-fighting" food. The ACS dietary guidelines for cancer prevention recommend achieving and maintaining a healthy weight throughout life. They also recommend that people:
- Choose foods, serving sizes, and caloric contents that promote a healthy weight.
- Eat at least 2 1/2 cups (590 grams) of fruits and vegetables each day.
- Choose whole grains instead of refined grain products.
- Limit consumption of processed and red meat.
- Limit alcohol consumption to 1 drink per day (women at high risk for breast cancer should consider not drinking alcohol at all).
For breast cancer survivors, the ACS recommends diets that include lots of fruits and vegetables, low amounts of saturated fat (from meat and high-fat dairy products), and moderate or no alcohol consumption.
Soy products contain isoflavones, which are a type of phytoestrogen. Phytoestrogens are plant compounds that have estrogen-like properties. Eating soy foods does not appear to be harmful for breast cancer survivors. However, it may be best to avoid concentrated amounts of isoflavones found in dietary supplements.
Specific Preventive Measures for High-Risk Women
Lifestyle Factors
Premenopausal women at higher risk, usually because of family history, should take as many preventive measures as possible, starting at an early age. In addition to regular physical activity, a healthy diet, and maintaining a healthy weight, the following lifestyle choices may be beneficial:
- Use alternatives to oral contraceptives and, if feasible, consider having children early in life
- Consider not taking hormone therapy
- Consider avoiding alcohol or drinking very sparingly
Preventive Medications (Tamoxifen and Raloxifene)
Drugs known as selective estrogen-receptor modulators (SERMs) act like estrogen in some tissues but behave like estrogen blockers (anti-estrogens) in others. Two SERMs, tamoxifen (Nolvadex, generic) and raloxifene (Evista), are approved for breast cancer prevention for high-risk women. Tamoxifen and raloxifene are not recommended as prevention for women at low or average risk for breast cancer.
For preventive purposes, the drugs are usually given as a pill taken daily for 5 years. Research indicates they may continue to provide protection for at least another 5 years after therapy ends.
Women at high risk for breast cancer should discuss with their doctors the risks and benefits of SERMs. Both of these drugs can cause hot flashes and increase the risks for blood clots. Tamoxifen is usually recommended as a first choice for premenopausal women. The aromatase inhibitor exemestane (Aromasin, generic) may be an alternative for postmenopausal women.
Preventive Surgery
Women who have a very high risk for breast cancer, due to factors such as BRCA genetic mutations or strong family history of breast cancer, may consider preventive (prophylactic) surgery. For these women, prophylactic mastectomy of both breasts can significantly reduce the risk of cancer. Prophylactic bilateral salpingo-oophorectomy (removal of both ovaries and fallopian tubes) can halve the risk for breast cancer and also significantly reduce the risk for ovarian cancer. Preventive surgery requires careful and serious consideration. Be sure to seek a second opinion from an oncologist before making a final decision.
Symptoms
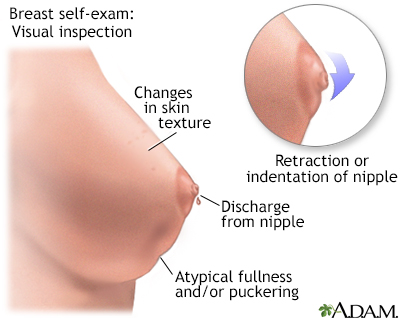
Breast cancers in their early stages are usually painless. Often the first symptom is the discovery of a hard lump in the breast or armpit. The lump may make the affected breast appear elevated or asymmetric. The nipple may be retracted (turned inwards) or scaly. Sometimes the skin of the breast is dimpled like the skin of an orange. In some cases there is a bloody or clear discharge from the nipple.
Women should keep aware of how their breasts look and feel. If you notice changes that persist, discuss your concerns with your health care provider. Some of these symptoms may be caused by conditions that are not cancer.
Many early breast cancers produce no symptoms and cannot be felt on examination. Mammogram screenings can help detect breast cancers before there are any symptoms.
Diagnosis
Breast Exams
Clinical Breast Exam
The American Cancer Society (ACS) recommends that women in their 20s and 30s have a clinical breast exam by a health professional every 3 years. The ACS also recommends that women over age 40 should be examined annually.
Breast Self-Exam and Breast Awareness
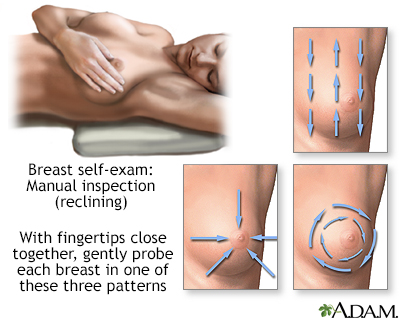
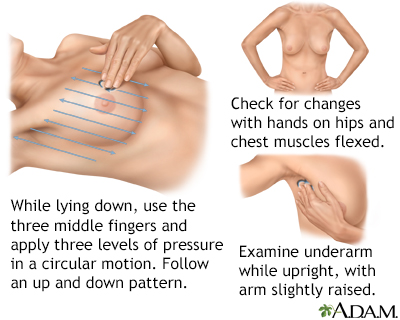
Women are encouraged to perform breast self-exams each month. However, the importance of self-exams for detecting breast cancer is debatable. Women who are not comfortable performing formal self-exams should at least try to practice breast awareness. This means knowing how your breasts normally look and feel so you can notice any changes that might occur. You can feel your breasts while taking a shower, and also inspect them periodically in a mirror.
Breast self-exams should include:
- Visual inspection (with and without a mirror) to note any changes in contour or texture.
- Manual inspection in standing and reclining positions to note any unusual lumps or thicknesses.
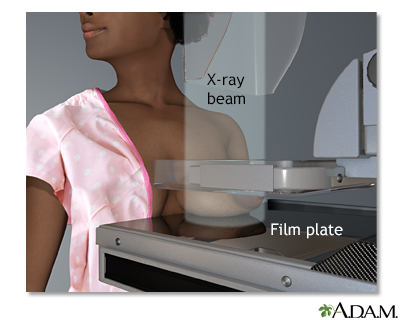
Mammograms
Mammography is a very effective screening method for breast cancer. It uses low-dose x-rays to take a picture (mammogram) of the breast. Mammograms can help catch breast cancer tumors while they are in their earliest and most treatable stages.
However, there is controversy about the value of mammography screening in preventing deaths from breast cancer. Mammograms in younger women produce a relatively high rate of false-positive results (when the test falsely indicates breast cancer) and may expose women to unnecessary radiation.
In addition, not all early-stage breast cancers become life-threatening. Women may undergo potentially unnecessary biopsies or surgeries. These issues have raised concerns on whether mammograms contribute to overdiagnosis and overtreatment.
While there is disagreement on the benefits of mammography for younger women, most experts agree that regular screening mammograms can help save lives in older women (over age 50), who are most at risk for breast cancer. Much of the debate over mammography focuses on when women should begin to have mammograms and how frequently they should receive them.
Current Recommendations for Screening
Most major professional groups, including the ACS and the American Congress of Obstetricians and Gynecologists, recommend that women have a mammogram every year, or every other year starting at age 50.
For women with average risk, The U.S. Preventive Services Task Force (USPSTF) recommends:
- For ages 40 to 49 years, the USPSTF does not recommend routine screening mammography. The decision to screen women in this age group should be made individually, on a case-by-case basis, taking a woman's values regarding specific benefits and harms into account.
- For ages 50 to 74 years, the USPSTF recommends that screening mammography be performed every other year.
Other organizations, such as the American Cancer Society, the American College of Obstetrics and Gynecology, and the American College of Radiology have recommendations that are different from those above. These recommendations may include routine screening beginning before age 50 as well as shorter intervals between screening tests.
Discuss the risks and benefits of mammography with your health care provider so you can make an informed decision. You should also take into consideration your personal risk profile.
Other Imaging Techniques
Magnetic Resonance Imaging (MRI)
The ACS recommends that women at high risk, such as those with BRCA genetic mutations or a strong family history of breast cancer, have an MRI of their breast performed in addition to a screening mammogram.
For women who have had cancer diagnosed in one breast, MRIs may also be helpful for detecting hidden tumors in the other breast. Breast MRI is a complicated procedure that requires special equipment and experienced radiologists. It should be performed at a facility that can also perform biopsies when suspicious findings are detected.
Ultrasound
Ultrasound is an imaging test that uses sound waves to create a picture (sonogram). Ultrasound of the breast is not used as a screening tool, but may be used if a mammogram or MRI suggests abnormalities. It can be used to help locate a mass in a biopsy procedure.
Biopsy
If a lump or other suspicious findings identified with ultrasound, mammography, or MRI are discovered, a biopsy will be performed. A biopsy is used to make a definitive diagnosis of breast cancer. A small piece of tissue is removed from the breast and sent to a laboratory to be examined under a microscope.
There are different techniques used to obtain a breast tissue sample:
- Fine needle aspiration biopsy uses a small thin needle attached to a syringe to take a sample of the lump.
- Core needle biopsy uses a tiny incision to insert a special needle to take samples.
- Vacuum-assisted biopsy uses a vacuum-powered needle.
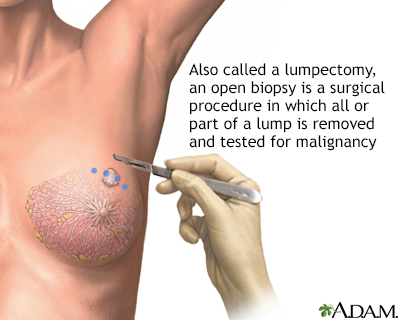
- Excisional (open) biopsy uses surgery to cut out and remove the mass.
Different imaging techniques may be used as guides to map the position of the mass to ensure that the abnormal tissue is correctly targeted:
- Ultrasound
- MRI
- Stereotactic (using mammography)
- Needle (wire) localization (using imaging to insert a metal wire into the suspected region; the surgeon uses the wire to locate and remove the mass)
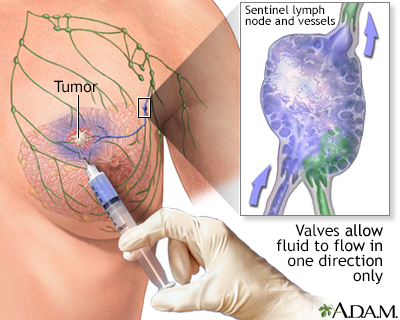
Sentinel Node Biopsy
The sentinel lymph node is the first lymph node that cancer cells are likely to spread to from the primary tumor (the original site of the cancer). Sentinel node biopsy is a procedure that examines the sentinel node to determine if cancer has spread.
Sentinel node biopsy involves:
- An injection of a tiny amount of a tracer, either a radioactively-labeled substance (radioisotope) or a blue dye, into the tumor site.
- The tracer or dye then flows through the lymphatic system into the sentinel node. This is the first lymph node to which any cancer would spread.
- The sentinel lymph node and possibly one or two others are then removed.
If they do not show any signs of cancer, it is highly likely that the remaining lymph nodes will be cancer free, making further surgery unnecessary.
Axillary Lymphadenectomy
If the sentinel node biopsy finds evidence that cancer has spread, the next diagnostic step is to find out how far it has spread. To do this, the doctor performs a procedure called an axillary lymphadenectomy, which partially or completely removes the lymph nodes in the armpit beside the affected breast (called axillary lymph nodes).
Once the lymph nodes are removed, they are analyzed to determine whether subsequent treatment needs to be more or less aggressive:
- If no cancer is found in the lymph nodes, the condition is referred to as node negative breast cancer. The chances are good that the cancer has not spread and is still local.
- If cancer cells are present in the lymph nodes, the cancer is called node positive. Their presence increases the possibility that the cancer has spread microscopically to other areas of the body.
- In node-positive cases, it is still not known if the cancer has metastasized beyond the lymph nodes or, if so, to what extent. The doctor may perform further tests to see if the cancer has spread to the bone (bone scan), lungs (x-ray or CT scan) or brain (MRI or CT scan).
Prognosis
Women are now living longer with breast cancer. In the United States, there are currently more than 2.8 million breast cancer survivors. Early detection and new treatments have improved survival rates.
Several factors are used to determine the risk for recurrence and the likelihood of successful treatment. They include:
- Stage of the cancer and how far it has spread
- Whether the tumor is hormone receptor-positive or -negative
- Tumor markers
- Gene expression
- Tumor size and shape
- Rate of cell division
Cancer Stage and Prognosis
Breast cancer survival rates are based in part on how advanced the cancer is when it is first diagnosed:
- If the cancer is ductal carcinoma in situ (DCIS) or is confined to the primary site (localized), 5-year survival rates are about 99%.
- If the cancer has spread to nearby lymph nodes (regional), the 5-year survival rate is somewhat reduced.
- If the cancer has spread to more distant sites (metastasized) such as the lung, liver, and bone, the 5-year survival rate is significantly reduced.
New drug therapies are helping to prolong survival for women with metastatic (stage IV) cancer.
Hormone Receptor-Positive or -Negative Breast Cancer
About two-thirds of breast cancer cells contain receptors, or binding sites, for the hormones estrogen and progesterone.
- Estrogen receptor-positive (ER-positive or ER+) breast cancer means that estrogen stimulates the growth of the cancer cells.
- Progesterone receptor-positive (PR-positive or PR+) means that progesterone stimulates the growth of the cancer cells.
- Breast cancer is considered hormone receptor-positive if the cells have receptors for one or both of these hormones. About 75% of all newly diagnosed breast cancers are ER+, PR+, or both.
- Breast cancer is considered hormone receptor-negative if the cells lack these receptors. About 25% of breast cancers are hormone receptor-negative.
Hormone receptor-positive cancer is also called "hormone sensitive" because it responds to hormone therapy such as tamoxifen or aromatase inhibitors. Hormone receptor-negative tumors are referred to as "hormone insensitive" or "hormone resistant."
Women have a better prognosis if their tumors are hormone receptor-positive because these cells grow more slowly than receptor-negative cells. In addition, women with hormone receptor-positive cancer have more treatment options.
HER2
The ACS recommends that all women newly diagnosed with breast cancer get a biopsy test for overexpression of a growth-promoting protein called HER2/neu. HER2-positive cancer usually occurs in younger women and is more quickly-growing and aggressive than other types of breast cancer. The HER2 marker is present in 15 to 20% of cases of invasive breast cancer.
Treatment with targeted therapy (biologic drugs) such as trastuzumab (Hercepin) may help women who test positive for HER2. A genetic test can help determine which people with HER2-positive breast cancer may be good candidates for this treatment.
Triple-negative breast cancer (TNBC)
Breast cancer is termed "triple-negative breast cancer" (TNBC) if the tumor lacks all three markers (ER, PR, and HER2/neu). Breast cancers associated with BRCA1 gene mutation are in many cases triple-negative.
The prevalence of TNBC is higher among African American compared to Caucasian women. TNBC is considered biologically aggressive and associated with a poor prognosis. Despite initial response to chemotherapy, TNBC is more likely to relapse early and to metastasize. Current trials are underway for new therapies targeting TNBC.
Gene Expression Profiling
Gene expression profiling tests (Oncotype DX, MammaPrint, others) examine a set of genes in tumor tissue to determine the likelihood of breast cancer recurrence. These tests are also used to help determine whether adjuvant (following surgery) drug treatments should be given for women with early-stage cancer. Based on the results, a doctor can decide whether a person who has had surgery may benefit from chemotherapy.
Other Factors for Predicting Outlook
Tumor Size
Large tumors pose a higher risk than small tumors.
Rate of Cell Division
The more rapidly a tumor grows, the more dangerous it is. Several tests are used to measure aspects of cancer cell division.
Treatment
The three main treatments of breast cancer are:
- Surgery
- Radiation
- Drug therapy
Most times, a combination of treatments is given. Treatment decision is determined by many factors, including:
- Age
- Menopausal status
- Cancer stage
- Hormone receptor and HER2 status
- Results of gene expression profiling tests
Some women may be candidates for clinical trials exploring new types or combinations of treatments.
Breast cancer treatments are defined as local or systemic:
- Local Treatment. Surgery and radiation are considered local therapies because they directly treat the tumor, breast, lymph nodes, or other specific regions. Surgery is usually the standard initial treatment.
- Systemic Treatment. Drug treatment is called systemic therapy, because it affects the whole body. Drugs may include chemotherapy, hormone therapy, and targeted therapy (biologic drugs).
Radiation and drug therapy are often given after surgery. This is called adjuvant therapy. The purpose of adjuvant therapy is to kill any cancer cells that remain after surgery. In some cases, drug therapy may be given before surgery (neoadjuvant therapy) to shrink the tumor and make it easier to surgically remove.
Cancer Stage and Treatment Options
Treatment strategies depend in part on the stage of the cancer. Breast cancer is staged based on:
- The size and location of the primary Tumor (T)
- If the tumor has spread to the lymph Nodes (N)
- If the cancer has spread (Metastasized) to other parts of the body (M)
The TNM system is used to classify cancer in stages I to IV. Each stage is further divided into substages.
Stage 0 (Carcinoma in Situ)
Stage 0 breast cancer is considered non-invasive ("in situ"), meaning that the cancer is still confined within breast ducts or lobules and has not yet spread to surrounding tissues. Stage 0 cancer is classified as either:
- Ductal carcinoma in situ (DCIS). These are cancer cells in the lining of a duct that have not invaded the surrounding breast tissue.
- Lobular carcinoma in situ (LCIS). These are cancer cells in the lobules of the breast. LCIS rarely develops into invasive breast cancer, but having it in one breast increases the risk of developing cancer in the other breast.
Treatment options for DCIS include:
- Breast-conserving surgery and radiation therapy (sometimes followed by hormone-blocking therapy for women with hormone-sensitive cancer). Many doctors recommend this approach.
- Total mastectomy
- Breast-conserving surgery without radiation therapy
Treatment options for LCIS include:
- Regular exams and mammograms to monitor any potential changes (observation treatment).
- Hormone-blocking therapy to prevent development of breast cancer (for women with hormone-sensitive cancer).
- Unlike DCIS, removal of LCIS with surgery or radiation is not indicated. The presence of LCIS indicated a 15% to 20% risk of developing invasive breast cancer, so some choose to have preventive (prophylactic) mastectomies. The surgery is not used to "treat" the LCIS, it is used to prevent a future diagnosis of invasive breast cancer.
Stage I and II (Early-Stage Invasive)
In stage I cancer, the tumor is no more than 2 cm (about 3/4 of an inch) across and has not spread to axillary lymph nodes (in the armpit) or distant sites. In stage II cancer the tumor may be larger than 2 centimeters and may have spread to lymph nodes.
Stage I and stage II treatment options may include:
- Breast-conserving surgery (lumpectomy) followed by radiation therapy.
- Modified radical mastectomy with or without breast reconstruction.
- Post-surgical therapy (adjuvant therapy) with radiation, chemotherapy, hormone-blocking therapy.
- Targeted therapy with trastuzumab (Herceptin) or with pertuzumab (Perjeta) given before surgery (neoadjuvant therapy) or after surgery (adjuvant therapy) together with chemotherapy for women with HER2-positive cancer.
Stage III (Locally Advanced)
In stage III, the cancer may have spread to lymph nodes within the breast or under the arm. It may have also spread to tissues near the breast, including the skin or chest wall.
Stage III treatment options may include:
- Surgery followed by chemotherapy, radiation, hormone therapy, and targeted therapy. Targeted therapy may also be used before surgery for women with HER2-positive cancer (as discussed above).
- Chemotherapy, and possibly hormone therapy (sometimes in combination with chemotherapy).
- Chemotherapy (with or without targeted therapy) followed by surgery (breast-conserving surgery or total mastectomy) with lymph node dissection followed by radiation therapy and possibly more chemotherapy or hormone-blocking therapy.
Stage IV (Advanced Cancer)
In stage IV, the cancer has spread (metastasized) from the breast and lymph nodes to other parts of the body. The most common metastasis sites are the bone, lung, and liver. The goals of treatment for stage IV cancer are to stabilize the disease and slow its progression, as well as to reduce pain and discomfort.
Stage IV treatment options may include:
- Chemotherapy, hormone therapy, or both.
- Targeted therapy in combination with chemotherapy or hormone therapy for women with HER2-positive cancer.
- Immunotherapy for patients with TNBC.
- Surgery or radiation to ease symptoms (palliative therapy).
- Drugs to prevent fractures if cancer has spread to the bone.
- Clinical trials of new drugs or combinations of treatments.
Recurrent Breast Cancer
Recurrent breast cancer is considered to be an advanced cancer. In such cases, the disease has come back in spite of the initial treatment. Most recurrences appear within the first 5 years after treatment, but breast cancer can recur many years later. Treatment options are based on:
- The stage at which the cancer reappears
- Whether or not the tumor is hormone responsive
- The age of the person
Women with recurrent breast cancer should discuss clinical trial options with their oncologists.
Post-Treatment Care
The American Society of Clinical Oncology (ASCO) recommends follow-up care for people who have been treated for breast cancer:
- Visit your doctor every 3 to 6 months for the first 3 years after your first cancer treatment, every 6 to 12 months during the fourth and fifth year, and once a year thereafter.
- Have a mammogram 1 year after the mammogram that diagnosed your cancer (but no earlier than 6 months after radiation therapy), and every 6 to 12 months thereafter.
- Perform a breast self-exam every month (however, this is no substitute for a mammogram).
- See your gynecologist regularly (women taking tamoxifen should be sure to report any vaginal bleeding).
- A year after diagnosis, you can either continue to see your oncologist or transfer your care to your primary care physician.
- If you are on hormone therapy, discuss with your oncologist how often to schedule follow-up visits for re-evaluation of your treatment.
- Genetic counseling may be helpful for some women including those with a family history of ovarian or breast cancer.
Breast cancer recurrences are often discovered by people in between doctor visits. It is important to notify your doctor if you experience any of the following symptoms in the years after treatment:
- New lumps in the breast
- Rash on breast
- Nipple discharge
- Swelling in lymph nodes under arm or collarbone
- Bone or chest pain
- Shortness of breath or difficulty breathing
- Persistent headaches or coughing
- Loss of appetite
Fertility and Pregnancy Treatment Considerations
There are no definite recommendations on how long a woman should wait to become pregnant after breast cancer treatment. Because of the connection between estrogen levels and breast cancer cell growth, some doctors recommend delaying pregnancy several months or years after treatment in order to reduce the risk of cancer recurrence and improve odds for survival. Discuss with your doctor your risk for recurrence, and when it may be safe to attempt pregnancy.
Women should discuss with their doctors any concerns and questions they may have about how cancer treatments could affect their fertility. They may also wish to have a consultation with a fertility specialist. Assisted reproductive technology such as embryo or oocyte (egg) cryopreservation ("freezing") may offer some women an option to later have children. ASCO recommends that you have these discussions with your health care team prior to starting cancer treatment.
Surgery
Surgery is a part of nearly every person's treatment for breast cancer. The initial surgical intervention is often a lumpectomy, the removal of the tumor itself. In the past, mastectomy (the removal of the entire breast) was the standard treatment for nearly all breast cancers. Now, many people with early-stage cancers can choose breast-conserving treatment.
Lumpectomy (Breast-Conserving Surgery)
Lumpectomy is the removal of the tumor plus a margin of normal tissue that surrounds it. This procedure is also called partial mastectomy or breast-conserving surgery.
You should discuss with your surgeon how much tissue may be removed. Some procedures remove only a small amount of tissue, while others (quadrantectomy) may remove about a quarter of your breast. In some cases, lymph nodes may also be removed.
If margins of the tissue removed show signs of cancer cells, the surgeon may need to perform another surgery to remove additional tissue. Radiation therapy administered after lumpectomy helps kill any cancer cells that may possibly remain. Research indicates that lumpectomy followed by radiation therapy is appropriate and as effective as mastectomy for most women with stage I or II breast cancers.
Side effects of lumpectomy vary depending on how much tissue was removed. They may include:
- Numbness
- Pain
- Arm or shoulder stiffness
You may need to wear a sports or supportive bra while healing to help prevent motion in your breast area that may cause pain. Your cancer care team should instruct you in arm exercises to help prevent stiffness.
Mastectomy
Mastectomy is surgery to remove the entire breast. There are different types of mastectomy:
- A total (simple) mastectomy involves removal of the whole breast. The surgeon does not remove lymph nodes under the armpit (axillary nodes) or muscle tissue. Sometimes lymph nodes are removed if they are in the area of the breast tissue.
- A modified radical mastectomy removes the breast and axillary lymph nodes, but no muscles.
- A radical mastectomy removes the breast, axillary lymph nodes, and chest wall muscles. This surgery is not performed as often as in the past.
- There are other mastectomy variations. These include nipple-sparing surgery and skin-sparing mastectomy. Skin-sparing mastectomy aims to retain as much of the outer skin of the breast as possible for implantation or breast reconstruction procedures.
After mastectomy you will stay in the hospital for several days. When you are discharged, your health care team will show you exercises to prevent arm and shoulder stiffness. You may feel stiffness and have difficulty extending your arm for a while.
The most frequent complication of extensive lymph node removal is lymphedema, or swelling of the arm on the same side where the breast was removed. Nerve damage may cause numbness or tingling sensations in the chest area. These side effects usually go away after several weeks but can last much longer.
Breast Reconstruction
After a mastectomy, some women choose breast prosthesis or opt for breast reconstruction, which can be performed at the time of the mastectomy itself, if desired.
The breast is reshaped using a saline implant or, for a more cosmetic result, a muscle flap is taken from elsewhere in the body. Muscle flap procedures are more complicated, however, and blood transfusions may be required. If the nipple is removed, it is rebuilt from other body tissues and color is applied using tattoo techniques. It is nearly impossible to rebuild a breast that is identical to its partner, and additional operations may be necessary to achieve a desirable effect.
Implants, including silicone implants, do not appear to put a woman at risk for breast cancer recurrence. However, breast implants are not lifetime devices. About half of women who receive an implant for breast reconstruction will need to have it removed or replaced about 10 years after implantation.
Radiation
Radiation therapy uses high-energy x-rays. It may be used after surgery (adjuvant) to kill cancer cells. Radiation therapy can help reduce the chance of breast cancer recurrence in the breast and chest wall. Radiation is also used in advanced stages of cancer for relief of symptoms and to slow progression.
Administration of Radiation Therapy
Radiation is generally administered in the following ways:
- External Beam Radiation. This type of radiation is delivered externally by an x-ray machine that targets radiation to the whole breast. If mastectomy was performed, it may be delivered to the chest wall. The treatment is usually given 5 days a week for about 6 weeks.
- Some hospitals offer a shortened course of 3 weeks of higher doses of radiation for people with early-stage breast cancer. This accelerated course is called hypofractionated radiation therapy and has shown good results for select people in several studies.
- Brachytherapy. Less commonly, radiation is delivered in implants (brachytherapy). Implants are most often used as a radiation boost after whole breast radiation.
Side Effects of Radiation Therapy
Short-term side effects of radiation can last for several months and may include:
- Fatigue
- Breast swelling and soreness
- Red and peeling skin (special creams can help)
Long-term side effects may include:
- Decreased size of breast
- Increased firmness of breast
- Skin redness and discoloration
- Swelling in the arm (lymphedema) in women who have had axillary lymph nodes removed
- In rare cases, rib fractures, heart problems, or development of second cancer
Current advanced imaging techniques use precise radiation that reduces exposure. These newer techniques are likely to reduce the risks for serious complications.
Drug Therapy
Drugs therapies for breast cancer include chemotherapy, targeted therapy, and hormone therapy:
- Chemotherapy drugs are "cytotoxic" (cell-killing) drugs. They are given orally or by injection. They work systemically by killing cancer cells throughout the body.
- Biologic drugs target proteins involved in cancer cell survival. Treatment with these drugs is called targeted therapy.
- Hormone therapy includes drugs that work to decrease estrogen, decrease the estrogen receptor, or modify estrogen receptor effects.
Chemotherapy needs to be tailored to the type of cancer involved. Women require different treatments depending on various factors including whether the tumor is hormone receptor-positive or -negative, or HER2-positive or -negative. Different treatment approaches are also used for early-stage cancer and advanced cancer.
Adjuvant chemotherapy is administered following surgery to kill remaining tumor cells. In some cases, neoadjuvant chemotherapy may be administered before surgery to help shrink the tumor.
Chemotherapy Drug Classes
Many different types of chemotherapy drugs are used to treat breast cancer. Common types of chemotherapy drug classes include:
- Anthracyclines include doxorubicin (Adriamycin, generic) and epirubicin (Ellence, generic).
- Taxanes include paclitaxel (Taxol, generic), albumin-bound paclitaxel (Abraxane), and docetaxel (Taxotere, generic). These drugs may be particularly helpful for node-positive breast cancer.
Chemotherapy Regimens for Early-Stage Breast Cancer
Some of the abbreviations used for chemotherapy drug combinations (regimens) refer to drug classes rather than drug names. For example, regimens that contain an anthracycline drug (such as doxorubicin) use the letter "A," and regimens that contain a taxane drug (such as docetaxel) use the letter "T."
Chemotherapy regimens usually consist of 4 to 6 cycles of treatment given over 3 to 6 months. Common chemotherapy regimens for early-stage breast cancer include:
- AC (Doxorubicin and cyclophosphamide)
- AC-T (Doxorubicin and cylophosphamide followed by paclitaxel)
- CAF (Cyclophosphamide, doxorubicin, and 5-FU)
- CMF (Cyclophosphamide, methotrexate, and 5-FU)
- TAC (Docetaxel, doxorubicin, and cyclophosphamide)
- FEC (5-FU, epirubicin, and cyclophosphamide)
For cancers that are HER2-positive, targeted therapy may be used. Targeted therapy uses biologic drugs that specifically target the HER2 protein on cancer cells. For early-stage HER2-positive breast cancer, targeted therapy drugs include:
- Trastuzumab (Herceptin) is given along with other chemotherapy drugs following surgery (adjuvant therapy). The treatment usually lasts for 1 year. Trastuzumab may also be given before surgery (neoadjuvant therapy) along with pertuzumab.
- Pertuzumab (Perjeta) is used before surgery (neoadjuvant therapy) or after surgery (adjuvant therapy) in combination with trastuzumab and chemotherapy.
Chemotherapy for Advanced (Metastatic) Cancer
Metastatic disease (cancer that spreads throughout the body) is generally not curable. New advances in drug therapies, however, can help:
- Shrink tumors
- Prolong survival
- Improve quality of life
Chemotherapy regimens for advanced cancer may use a single drug or a combination of drugs. Different treatments are used depending on whether a woman has HER2-negative or HER2-positive cancer.
For HER2-negative advanced breast cancer, the drug therapy approach may follow the below treatments:
- Endocrine therapy as first-line treatment for most women with estrogen receptor-positive advanced cancer (see Hormone Therapy section)
- Chemotherapy drugs used singly and sequentially. A taxane or anthracycline drug is the first choice. Combination chemotherapy is not recommended except for life-threatening circumstances.
- Recently, the FDA approved a new class of targeted drug therapy for the treatment of advanced hormone receptor-positive, HER2-negative breast cancer. Cyclin-dependent kinases 4/6 (cdk4/6) inhibitors target a crucial enzyme that regulates the cell cycle. Currently, there are three cdk4/6 inhibitors on the market: palbociclib (Ibrance), FDA-approved in 2015, ribociclib (Kisqali), FDA-approved in 2017, and abemaciclib (Verzenio), FDA-approved in 2018. These drugs are used in combination with aromatase inhibitors such as letrozole (Femara) or with selective estrogen receptor degraders such as fulvestrant (Faslodex).
- The addition of targeted therapy with bevacizumab (Avastin) chemotherapy is controversial. It does not improve overall survival. (This drug is not FDA-approved for treating breast cancer.)
- Women with advanced breast cancer should consider enrolling in a clinical trial.
For HER2-positive advanced breast cancer, treatment recommendations include:
- Targeted therapy with biologic drugs is recommended.
- First-line therapy is a combination of trastuzumab (Herceptin), pertuzumab (Perjeta), and chemotherapy with a taxane drug.
- Second-line option is the targeted therapy trastuzumab emtansine (Kadcyla), which is approved for women who were previously treated with trastuzumab and a taxane chemotherapy drug
- Third-line options may include the use of the targeted therapy drug lapatinib (Tykerb), chemotherapy, and hormone therapy.
Side Effects of Chemotherapy
Side effects occur with all chemotherapy drugs. They are more severe with higher doses and increase over the course of treatment. Specific side effects depend on the drug, or drugs, used.
Common side effects of chemotherapy may include:
- Nausea and vomiting
- Diarrhea
- Temporary hair loss
- Mouth sores
- Changes in appetite
- Fatigue
More serious complications, which may be short-term or long-term, include:
- Shortness of breath due to low red blood cell count (anemia).
- Increased risk for bruising from low blood platelet counts (thrombocytopenia).
- Increased chance for infection from drop in white blood cell counts (neutropenia).
- Premature menopause and infertility.
- Problems with concentration and memory ("chemo brain").
- Nerve damage and pain.
- Heart problems. Trastuzumab (Herceptin) may increase the risk for heart failure, particularly in women with pre-existing risk factors. Cumulative doses of anthracyclines (doxorubicin, epirubicin) can also damage heart muscles over time and increase the risk for heart failure.
Targeted Therapy
Targeted therapy uses biologic drugs that target specific proteins that are important for cancer cell proliferation or spreading. As mentioned above, these drugs are used together with chemotherapeutics as adjuvants, neoadjuvants, or in metastatic breast cancer. The targeted therapies currently approved by for breast cancer include:
- Trastuzumab (Herceptin) and pertuzumab (Perjeta) are antibodies that target the HER2 protein on breast cancer cells. These are used for HER2-positive breast cancer.
- Lapatinib (Tykerb) and neratinib (Nerlynx) target tyrosine kinase and may be used for the treatment of HER2-positive breast cancer.
- Palbociclib (Ibrance), ribociclib (Kisqali), and abemaciclib (Verzenio) target cyclin-dependent kinases 4/6. These may be used for hormone receptor-positive, HER2-negative advanced breast cancer, in combination with other drugs.
- Trastuzumab emtansine or T-DM1 (Kadcyla) is an antibody-chemotherapy conjugate drug which combines targeting HER2 by the antibody trastuzumab (T) with the cytotoxic effects of emtansine (or DM1). T-DM1 is approved for advanced HER2-positive breast cancer that is resistant to trastuzumab alone.
- Everolimus (Afinitor) targets mTOR and is approved in combination with aromatase inhibitors for the treatment of postmenopausal women with hormone receptor-positive, HER2-negative breast cancer.
- Alpelisib (Piqray) targets PIK3CA-mutated breast cancer. It is approved in combination with fulvestrant in hormone receptor positive cancer.
- Olaparib (Lynparza) and talazoparib (Talzenna) is a PARP inhibitor that is approved for the treatment of BRCA-mutated breast cancer.
- Atezolizumab (Tecentriq), targeting the interaction between cancer cells and the immune system, was FDA-approved in 2019 for the treatment of TNBC overexpressing the PD-L1 protein. This drug is approved in combination with paclitaxel (Taxol).
Hormone Therapy
The goal of hormone therapy is to prevent estrogen from stimulating proliferation of breast cancer cells. It is recommended for people whose breast cancers are hormone-receptor positive (hormone sensitive). Like chemotherapy, hormone therapy works systemically. Hormone therapy is usually given after surgery (adjuvant) but may also be used in some cases before surgery (neoadjuvant).
Different types of hormone therapy work in different ways by:
- Blocking estrogen receptors in cancer cells (tamoxifen)
- Stimulating the degradation of the estrogen receptor (fulvestrant)
- Suppressing conversion of testosterone to estrogen in the body (aromatase inhibitors)
- Suppressing the ovaries, which produce estrogen (ovarian ablation)
Tamoxifen and Selective Estrogen Receptor Modulators (SERMs)
Tamoxifen (Nolvadex, generic) has been the standard hormonal drug used for breast cancer. It belongs to a class of compounds called selective estrogen receptor modulators (SERMs). SERMs chemically resemble estrogen and trick the breast cancer cells into accepting it in place of estrogen. Unlike estrogen, however, they do not stimulate breast cancer cell growth. Because SERMs block estrogen's effects on cancer cells, they are sometimes referred to as "anti-estrogen" drugs.
Tamoxifen is used for all cancer stages in (mainly premenopausal) women with hormone receptor-positive cancers. In addition, it is used to prevent breast cancer in high-risk women.
To prevent cancer recurrence, women should take tamoxifen for a total of 10 years following surgery. The American Society of Clinical Oncology (ASCO) recommends that women who are premenopausal or perimenopausal should start with tamoxifen for 5 years. If at that point they have still not reached menopause, they can continue with tamoxifen. If they have become postmenopausal, they can choose between tamoxifen and an aromatase inhibitor.
Tamoxifen is an effective cancer treatment, but it can cause unpleasant side effects and has small (less than 1%) but serious risks for blood clots and uterine (endometrial) cancer. Immediately report any signs of vaginal bleeding to your doctor, as this may be a symptom of uterine cancer. Tamoxifen risks for blood clots may be higher for women who are obese.
Less serious, but discomforting, side effects include hot flashes and mood swings. Some women want to stop treatment because of these side effects. However, stopping tamoxifen treatment prematurely can increase the risk for cancer recurrence and death. Talk with your cancer care team about therapies that may help you cope with side effects.
Selective estrogen receptor degraders (SERDs)
SERDs work by binding to the estrogen receptor and destabilizing it, such that it becomes a target for the protein degradation machinery of the breast cancer cell. The only FDA-approved SERD to date is fulvestrant (Faslodex). Fulvestrant is used for hormone receptor-positive advanced breast cancer in postmenopausal women in whom tamoxifen or aromatase inhibitors no longer work. Fulvestrant was also recently approved for use in combination with the cdk4/6 inhibitor palbociclib (Ibrance) for hormone receptor-positive, HER2-negative advanced breast cancer.
Aromatase Inhibitors
Aromatase inhibitors are recommended for postmenopausal women with hormone-sensitive breast cancer. Aromatase inhibitors are taken for up to 5 years. They can be used either before or after tamoxifen treatment.
Aromatase inhibitors block aromatase, an enzyme that is a major source of estrogen in many major body tissues, including:
- Breast
- Muscle
- Liver
- Fat
Aromatase inhibitors work differently than SERMs and SERDs. Aromatase inhibitors reduce the overall amount of estrogen in the body by blocking conversion of testosterone to estrogen by the enzyme aromatase.
Because these drugs cannot stop the ovaries of premenopausal women from producing estrogen, they are recommended only for postmenopausal women.
Three aromatase inhibitors are approved for treating early-stage, hormone receptor-positive breast cancer in postmenopausal women:
- Anastrazole (Armidex and generic)
- Exemestane (Aromasin and generic)
- Letrozole (Femara and generic)
There are no significant differences between these three drugs. People who cannot tolerate one type of aromatase inhibitor can switch to a different one. Studies indicate that the introduction of aromatase inhibitors has helped greatly in prolonging survival for women with advanced cancer.
Like tamoxifen, aromatase inhibitors can cause hot flashes. These drugs can also cause joint pain. Compared to tamoxifen, aromatase inhibitors are less likely to cause serious problems like blood clots and uterine cancer. However, they are more likely to cause osteoporosis, which can lead to fractures. Women should have their bone mineral density monitored during aromatase inhibitor treatment.
Ovarian Ablation
Ovarian ablation is a treatment that stops estrogen production from the ovaries. Medications can accomplish ovarian ablation. Destroying the ovaries with surgery or radiation can also shut down estrogen production. (Osteoporosis is one serious side effect of this approach, but can be treated with drug therapies.)
Chemical Ovarian Ablation
Drug treatment to block ovarian production of estrogen is called chemical ovarian ablation. It is often reversible. The primary drugs used are luteinizing hormone-releasing hormone (LHRH) agonists, such as goserelin (Zoladex). (They are also sometimes called GnRH agonists). These drugs block the release of the reproductive hormones LH-RH, therefore stopping ovulation and estrogen production.
Bilateral Oophorectomy
Bilateral oophorectomy, the surgical removal of both ovaries, is a surgical method of ovarian ablation. It may modestly improve breast cancer survival rates in some premenopausal women whose tumors are hormone receptor-positive. In these women, combining this procedure with tamoxifen may improve results beyond those of standard chemotherapies. Oophorectomy does not benefit women after menopause. The procedure causes sterility.
Resources
- National Cancer Institute -- www.cancer.gov
- American Cancer Society -- www.cancer.org
- American Society of Clinical Oncology -- www.asco.org
- BreastCancer.Org -- www.breastcancer.org
- Susan G. Komen Breast Cancer Foundation -- www.komen.org
- National Comprehensive Cancer Network -- www.nccn.org
- Cancer.Net -- www.cancer.net
- Find clinical trials -- www.cancer.gov/clinicaltrials
References
American Cancer Society website. American Cancer Society Recommendations for the early detection of breast cancer. www.cancer.org/cancer/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html. Updated October 3, 2019. Accessed December 17, 2019.
Andre F, Ismaila N, Stearns V. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO Clinical Practice Guideline Update Summary. J Oncol Pract. 2019; 15(9):495-497. PMID: 31306037 www.ncbi.nlm.nih.gov/pubmed/31306037.
Antoniou AC, Casadei S, Heikkinen T, et al. Breast-cancer risk in families with mutations in PALB2. N Engl J Med. 2014;371(6):497-506. PMID: 25099575 www.ncbi.nlm.nih.gov/pubmed/25099575.
Burstein HJ, Lacchetti C, Griggs JJ. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO Clinical Practice Guideline Focused Update. J Oncol Pract. 2019;15(2):106-107. PMID: 30523754 www.ncbi.nlm.nih.gov/pubmed/30523754.
Centers for Disease Control and Prevention (CDC). Vital signs: racial disparities in breast cancer severity - United States, 2005-2009. MMWR Morb Mortal Wkly Rep. 2012;61(45):922-926. PMID: 23151952 www.ncbi.nlm.nih.gov/pubmed/23151952.
Centers for Disease Control and Prevention (CDC). What is breast cancer screening? www.cdc.gov/cancer/breast/basic_info/screening.htm. Updated July 25, 2023. Accessed November 28, 2023.
Esteva FJ, Hubbard-Lucey VM, Tang J, Pusztai L. Immunotherapy and targeted therapy combinations in metastatic breast cancer. Lancet Oncol. 2019;20(3):e175-e186. PMID: 30842061 www.ncbi.nlm.nih.gov/pubmed/30842061.
Giordano SH, Temin S, Davidson NE. Systemic therapy for patients with advanced human epidermal growth factor Receptor 2-Positive Breast Cancer: ASCO Clinical Practice Guideline Update Summary. J Oncol Pract. 2018;14(8):501-504. PMID: 29989839 www.ncbi.nlm.nih.gov/pubmed/29989839.
Goetz MP, Gradishar WJ, Anderson BO, et al. NCCN Guidelines Insights: Breast cancer, Version 3.2018. J Natl Compr Canc Netw. 2019;17(2):118-126. PMID: 30787125 www.ncbi.nlm.nih.gov/pubmed/30787125.
Henry NL, Shah PD, Haider I, Freer PE, Jagsi R, Sabel MS. Cancer of the breast. In: Niederhuber JE, Armitage JO, Kastan MB, Doroshow JH, Tepper JE, eds. Abeloff's Clinical Oncology. 6th ed. Philadelphia, PA: Elsevier; 2020:chap 88.
Lyman GH, Temin S, Edge SB, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: 2016 American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;13(3):196-198. PMID: 28118104 www.ncbi.nlm.nih.gov/pubmed/28118104.
Moyer VA. Medications for risk reduction of primary breast cancer in women: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2013;159(10):698-708. PMID: 24061412 www.ncbi.nlm.nih.gov/pubmed/24061412.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast cancer. Version 2.2019. www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Updated July 2, 2019. Accessed August 15, 2019.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast cancer risk reduction. Version 1.2019. www.nccn.org/professionals/physician_gls/PDF/breast_risk.pdf. Updated December 11, 2018. Accessed August 15, 2019.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ®) Breast cancer screening and diagnosis Version 1.2019. www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf. Updated May 17, 2019. Accessed August 15, 2019.
Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2018;36(19):1994-2001. PMID: 29620997 www.ncbi.nlm.nih.gov/pubmed/29620997.
Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J Clin. 2016;66(1):43-73 PMID: 26641959 www.ncbi.nlm.nih.gov/pubmed/26641959.
Siu AL, US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164(4):279-296. PMID: 26757170 www.ncbi.nlm.nih.gov/pubmed/26757170.
U.S. Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: U.S. Preventive Services Task Force recommendation statement. Am Fam Physician. 2015;91(2):Online. PMID: 25591222 www.ncbi.nlm.nih.gov/pubmed/25591222.
Valle LF, Agarwal S, Bickel KE, Herchek HA, Nalepinski DC, Kapadia NS. Hypofractionated whole breast radiotherapy in breast conservation for early-stage breast cancer: a systematic review and meta-analysis of randomized trials. Breast Cancer Res Treat. 2017;162(3):409-417. PMID: 28160158 www.ncbi.nlm.nih.gov/pubmed/28160158.
Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(23):2942-2962. PMID: 23835710 www.ncbi.nlm.nih.gov/pubmed/23835710.
Review Date: 9/4/2019
Reviewed By: Todd Gersten, MD, Hematology/Oncology, Florida Cancer Specialists & Research Institute, Wellington, FL. Review provided by VeriMed Healthcare Network. Also reviewed by David Zieve, MD, MHA, Medical Director, Brenda Conaway, Editorial Director, and the A.D.A.M. Editorial team.